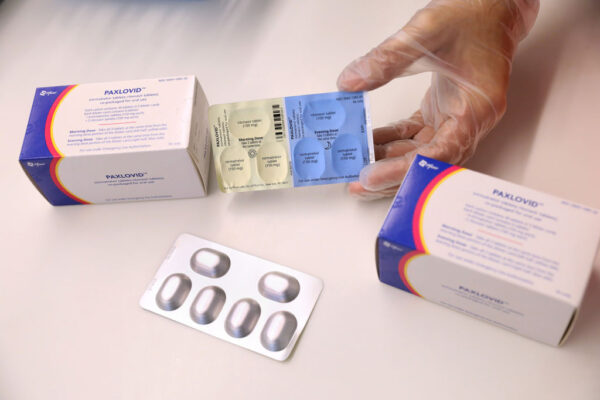
Pfizer’s Paxlovid now has full FDA approval for treating mild-to-moderate Covid-19, a regulatory decision that makes the pill the first oral antiviral for the novel coronavirus.
The approval announced Thursday covers the treatment of adults who are at high risk of progressing to severe Covid-19 that could lead to hospitalization or death. Paxlovid’s emergency authorization in late 2021 covered adults as well as children 12 and older who weigh more than 88 pounds.
Eligible children who need Paxlovid will still have access to it. The FDA said that Paxlovid manufactured and packaged under emergency use authorization and distributed by the U.S. Department of Health and Human Services will continue to be available for adults and children ages 12 to 18 who are not covered by the new approval. Neither the authorization nor the latest approval cover the prevention of Covid-19 infection.
Paxlovid is a small molecule protease inhibitor. The drug is designed to block Mpro, an enzyme that’s key in the viral replication cycle. Patients must take Paxlovid with ritonavir, which slows the breakdown of Paxlovid. Ritonavir keeps Paxlovid in the body longer and at higher concentrations.
FDA approval of Paxlovid was based on the final analysis of a placebo-controlled Phase 2/3 that enrolled non-hospitalized adults with a laboratory-confirmed diagnosis of SARS-CoV-2 infection. Those participants also had to have either a risk factor for progression to severe disease or be 60 years of older. Additionally, the trial participants had not previously been vaccinated for Covid-19 nor had they been previously infected by the virus.
Study results showed that compared to a placebo, treatment with Paxlovid led to an 86% reduction in Covid-19-related hospitalization or death from any cause. The Pfizer drug regimen started within five days of the onset of symptoms and patients were followed for 28 days after dosing. In the final analysis, 977 patients received Paxlovid while 989 study participants received a placebo. The FDA said 0.9% of those treated with the Pfizer drug Paxlovid were hospitalized due to Covid-19 or died from any cause during the 28 day follow-up period compared to 6.5% of those who received a placebo.
In the Phase 2/3 study as well as a separate Phase 3 study that enrolled vaccinated participants with at least one risk factor for progression to severe Covid-19, the FDA said there was a reduction in the risk of hospitalization from Covid-19 or death from any cause. But that reduction was not statistically significant.
“Today’s approval demonstrates that Paxlovid has met the agency’s rigorous standards for safety and effectiveness, and that it remains an important treatment option for people at high risk for progression to severe Covid-19, including those with prior immunity,” Patrizia Cavazzoni, director for the FDA’s Center for Drug Evaluation and Research, said in a prepared statement. “The FDA remains committed to working with sponsors to facilitate the development of new prevention and treatment options for Covid-19.”
One of the concerns surrounding Paxlovid is the observation of a “rebound,” in which patients apparently recover only to develop Covid-19 symptoms and then test positive again. In both studies, a rebound developed in a subset of patients, an effect observed in both the treatment arm and the placebo group. The FDA said there is no clear association between treatment with Paxlovid and Covid-19 rebound.
Paxlovid’s label carries a black box warning that cautions physicians and patients about dangerous drug interaction risks. Ritonavir’s role of enhancing the effect of Paxlovid can also lead other drugs to have greater exposure, which could in turn lead to life-threatening effects. The label directs prescribers to review all medications taken by a patient to assess the potential of a drug interaction and to determine if doses of those drugs need to be adjusted or interrupted.
Paxlovid accounted for $18.9 billion in 2022 revenue, $10.5 billion of which came from the U.S., according to Pfizer’s annual report. The drug was mainly sold to government agencies. As those contracts expire, the pharmaceutical giant expects to transition sales to commercial channels in the second half of this year, the report said. Consequently, sales are not expected to reach 2022 levels. While Pfizer still expects strong demand for its Covid-19 products this year, much of that demand will be fulfilled by supplies delivered to governments and recorded as revenue last year. The company projects Paxlovid revenue will be about $8 billion in 2023, down 58% from 2022.
The affirmatory decision for Paxlovid makes it the fourth FDA-approved drug for treating Covid-19. The others are Gilead Sciences’ Veklury, Actemra from Roche, and Olumiant from Eli Lilly. Like Paxlovid, Veklury is an antiviral intended to stop viral replication. Actemra, an infusion, and Olumiant, dosed as a pill, work by stopping the body’s inflammatory responses to the virus.
Photo: Chris Sweda/Chicago Tribune/Tribune News Service, via Getty Images